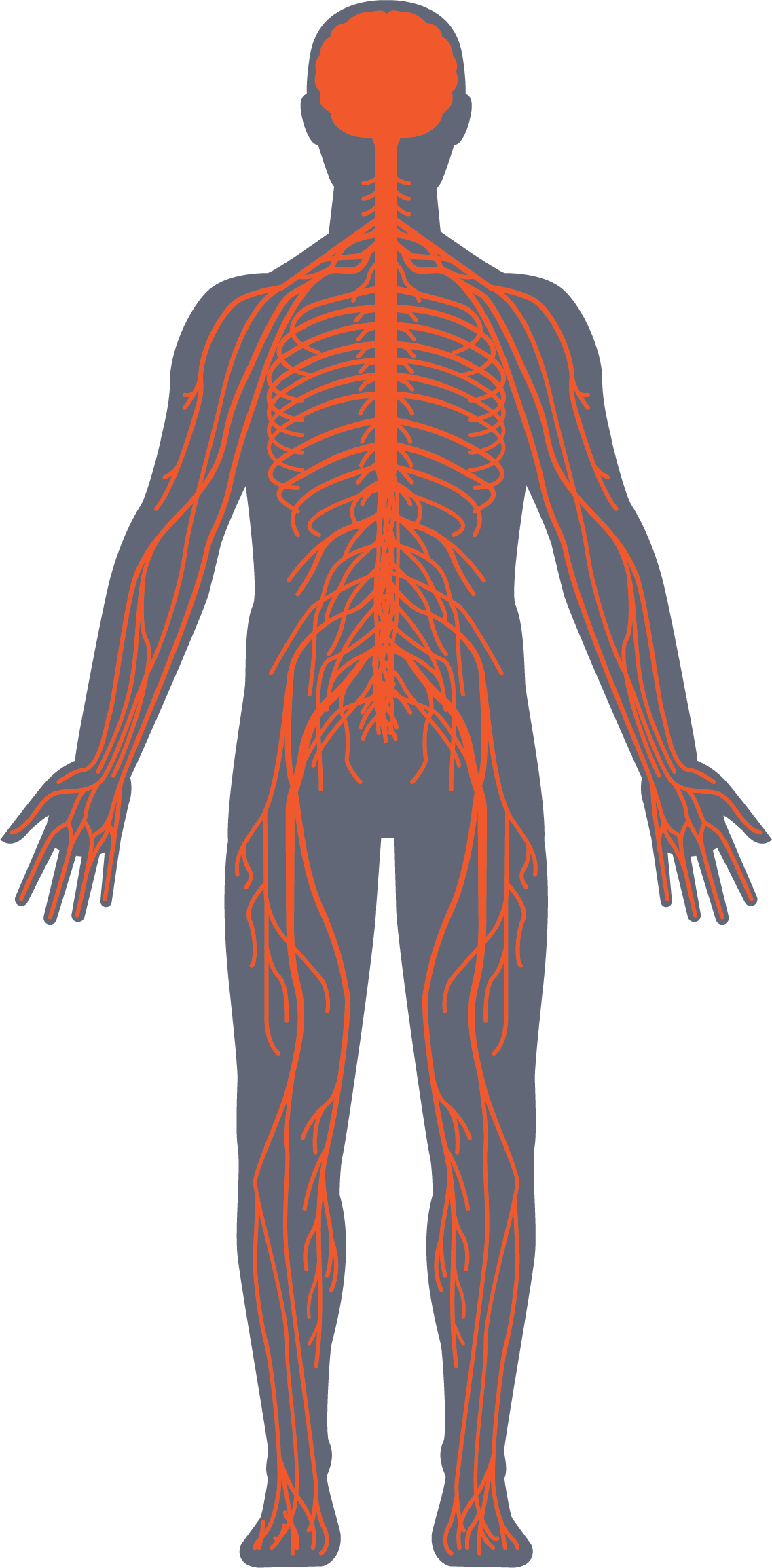
Innovating nerve repair
Improving patient
outcomes
Cleaner. Safer. Better.
About ReNerve
ReNerve specializes in developing a range of ‘ready to use’ products that are cleaner, safer and better for the repair or replacement of damaged peripheral nerves resulting in better patient outcomes.
ReNerve, a medical device company, founded by a neurosurgeon and medtech researchers focusing on customised nerve replacement products for specific nerve replacement procedures. Focusing on the innovation and customisation of nerve repair in nerve surgery.

ReNerve Product Development

NervAlign® Nerve Cuff
Transected nerves with no gap. Synthetic conduits, grafts or donor tissue.
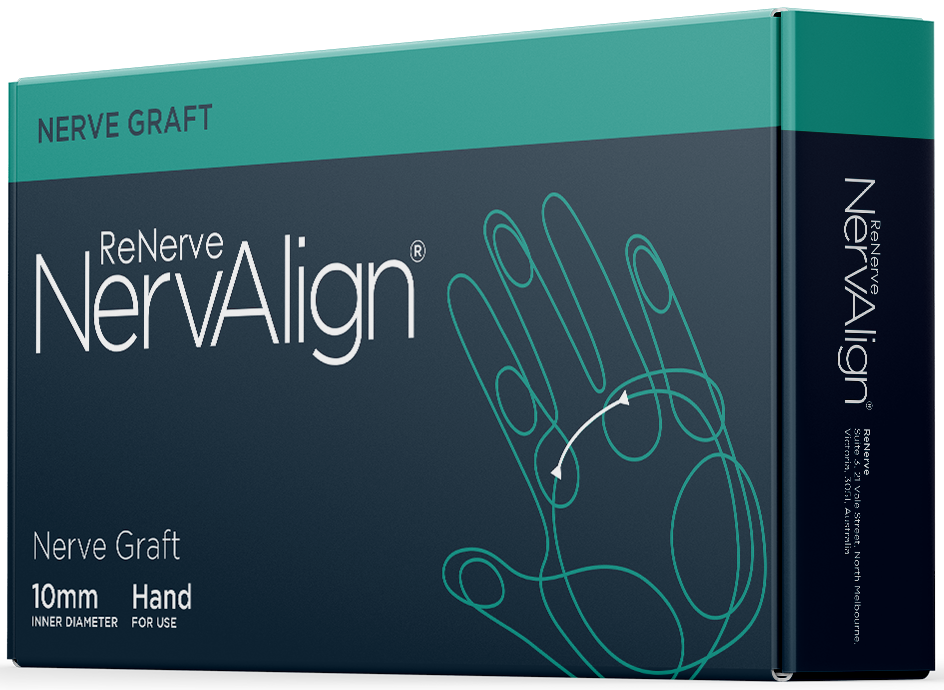
NervAlign® Nerve Graft
For transected long gap. An off-the-shelf, ready to use nerve structure to replace the most challenging repair.

NervAlign® Bionic Nerve
For transected long gap. An off-the-shelf, ready to use nerve structure to replace the most challenging repair.
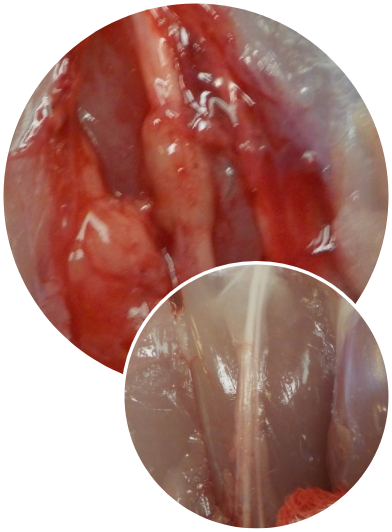

The Science
The NerveAlign® product range is designed to provide a supportive interface that facilitates precise surgical technique and may help mitigate patient discomfort following repair.
Partnerships
ReNerve’s propriety technologies offer an ideal platform for collaborative programs to innovate and develop new products and solutions for peripheral nerve repair. We would welcome opportunities for further discussions.
